-
Earth Science Journal!
#19 The Heating "Curve" of Water
I have some small cups of frozen water in the freezer.
What temperature do you think the ice will be when taken out of the freezer?
Place your prediction down in Celsius and use your reference tables to convert it to Fahrenheit.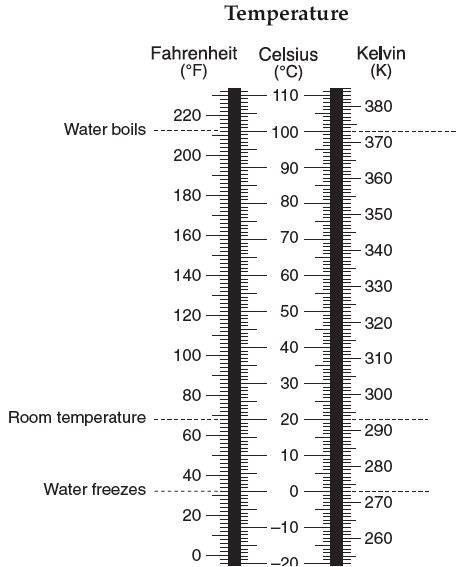
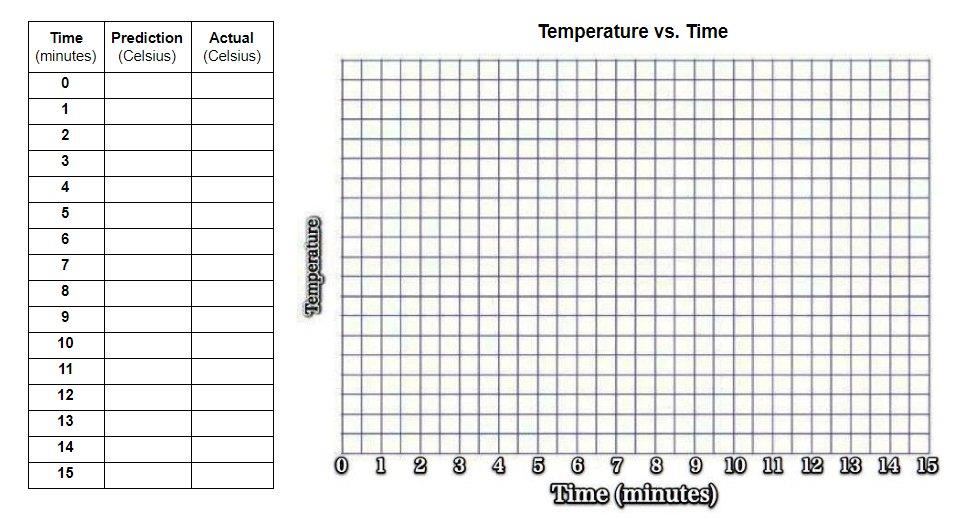
The actual temperature of the ice in the cups (time zero). ________°C
Make all your predictions in degrees Celsius.
Based on the actual temperature, predict the ice's temperature after one minute.
Let's find out the current results for minute one.
Predict what the temperature will be for minute two.
Let's find out the actual results for minute two.
Minutes three, four...
Based on our observations of the first few minutes, predict (infer) the temperature through minute 15.
Plot your prediction for the ice heated by the room.
You will need a temperature scale on the Y-axis, starting with the actual temperature of the ice at time zero and increasing by 1 degree Celsius.
Now, let's find out the actual temperatures.
- How accurate were your predictions?
- What do you notice about the results?
Plot the actual temperature of the ice heated by the room.
Use two different colored or labeled lines.
Rate of heating for the ice sitting on the desk.
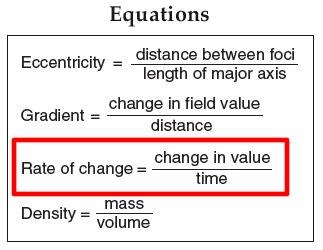
3. Calculate the rate of change for ice sitting on the desk from time zero until it reaches zero degrees Celsius. Round your answers to the nearest tenth and include proper units.
Rate = ___________________ =
4. What were some heat sources and heat sinks in this demonstration?
Heat Sources: the room (air, desk) and handsHeat Sinks: the ice, the plastic cups, the thermometer
Use your earth science reference tables to answer the following questions.
5. At what temperature does water freeze? __ 32°Fahrenheit ___ 0°Celsius _ 273 Kelvin
Note: At what temperature does ice melt? 32°Fahrenheit 0°Celsius 273 Kelvin
6. At what temperature does water boil? 212°Fahrenheit 100°Celsius 373 kelvin
Specific Heat (front page of your reference tables)
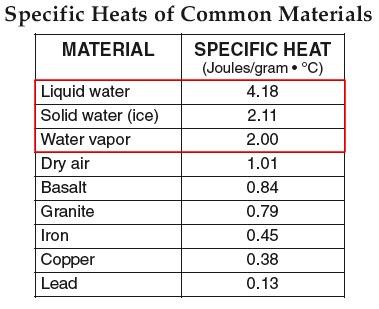
7. How many Joules of energy does it take to raise 1 gram of water by 1 degree Celsius? 4.18 Joules
8. How many Joules of energy does it take to raise 1 gram of ice by 1 degree Celsius? 2.11 Joules
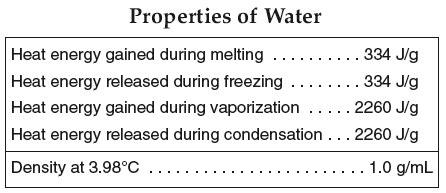
9. How many Joules of energy are needed to melt 1 gram of ice? 334 Joules
10. How many Joules of energy are needed to evaporate 1 gram of water? 2260 Joules
11. Is heat released or gained during condensation? Released
12. What will happen when a weighted wire (copper, fishing line) is set on top of ice?13. Where did the energy come from for the wire to melt the ice?
Answer: It came from the energy released during refreezing just above the wire.14. Why do metals feel colder or hotter than other materials of the same temperature?
They feel colder because metals have a low specific heat, and heat can transfer more quickly from the metal to your hand, making it feel cold.
Properties of water (reference tables)

15. What happens to the temperature of the air when water droplets condense, creating clouds?
Cloud formation heats the surrounding air just as the freezing ice heated the copper wire.
16. What takes more energy, raising 100 grams of liquid water by 10 degrees Celsius, melting 10 grams of ice, or evaporating 2 grams of water?

Answer: Evaporating just 2 grams of water!

17. What temperature is water most dense? About 4 degrees Celsius above zero!
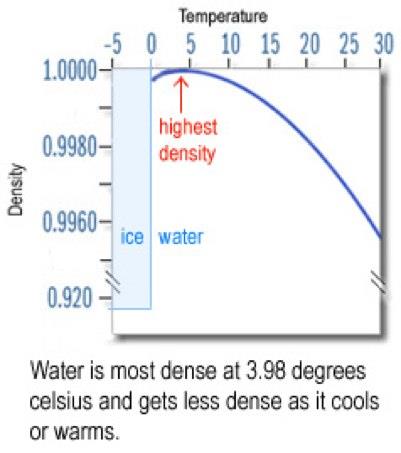 That means that the bottom of Lake Ontario is warmer than the top of Lake Ontario when people go Polar Bear swimming!
That means that the bottom of Lake Ontario is warmer than the top of Lake Ontario when people go Polar Bear swimming!
Circle the Density at 3.98°C
Place the following terms on your graph.
Water, steam (water vapor), ice, melting, boiling (evaporation), water (heating/cooling),
ice (heating/cooling, steam (heating/cooling), 0°C, 212°F, 32°F, 100°C -Don't forget to break the line for evaporation/condensation.Tape this in your journal.
-Don't forget to break the line for evaporation/condensation.Tape this in your journal.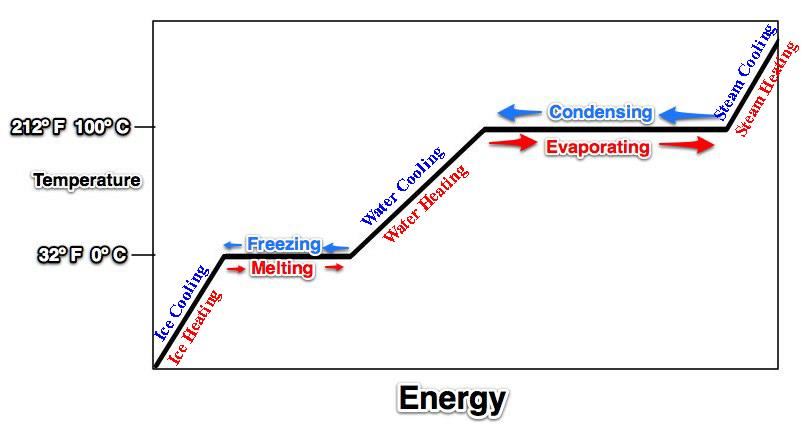 Now, let's place the energy of specific and latent heat values on our graph.
Now, let's place the energy of specific and latent heat values on our graph.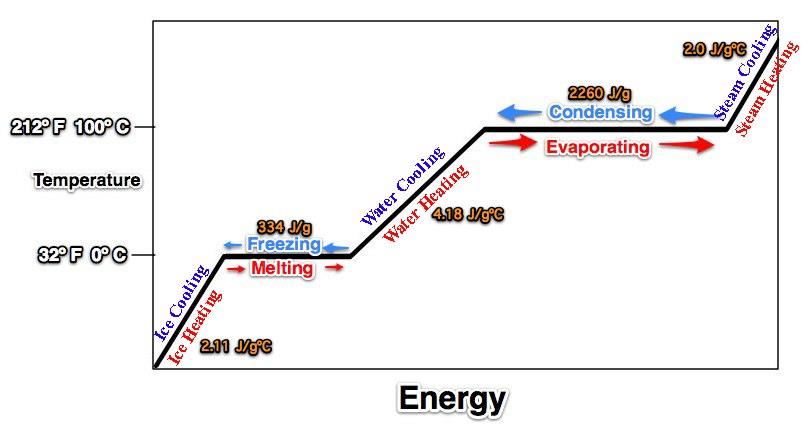
18. According to the graph, what heats faster: solid ice, liquid water, or water vapor?
How do you know?
Water Vapor because it has the lowest specific heat (steepest line)Practice Quiz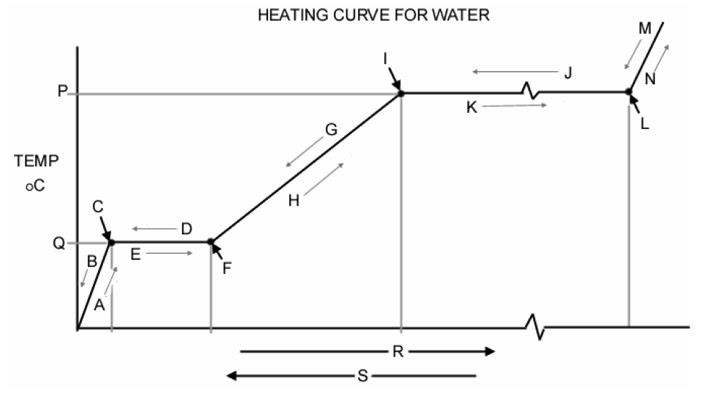 Answer the following based on the graph above.1. Which letter represents ice getting warmer? Letter A2. Which letter represents liquid water at 100º C? Letter I3. Which letter represents ice at 0º C? Letter C4. The process of evaporation (vaporization) is indicated by the arrow labeled with the letter Letter K5. Which of the following processes (melting, evaporation, condensation, freezing) involves the GREATEST release of energy? Condensation!6. Which letter represents liquid water heating? Letter H7. What is the temperature in degrees Celsius at point P? 100 degrees Celsius8. What is the name for the process indicated by the letter J? Condensation
Answer the following based on the graph above.1. Which letter represents ice getting warmer? Letter A2. Which letter represents liquid water at 100º C? Letter I3. Which letter represents ice at 0º C? Letter C4. The process of evaporation (vaporization) is indicated by the arrow labeled with the letter Letter K5. Which of the following processes (melting, evaporation, condensation, freezing) involves the GREATEST release of energy? Condensation!6. Which letter represents liquid water heating? Letter H7. What is the temperature in degrees Celsius at point P? 100 degrees Celsius8. What is the name for the process indicated by the letter J? Condensation
Reflection: Explain why the temperature did not rise as many people predicted in the ice cups.
Bonus: If, when things get colder, their molecules move slower, and they become more compact (dense), so why does ice float? How would the world be different if ice sank?

