-
Journal Entry #3
Demonstrating Density!
Introduction: Students will be able to explain the concept of density and solve density problems by watching a demo, taking notes, and doing practice problems.
Vocabulary
Mass: The amount of matter in an object
Volume: The amount of space an object occupies
Density: The concentration of matter in an objectNote: Even though we determine an object's mass by weighing it on a scale, mass differs from weight, which depends on gravity. Since the force of gravity is very consistent on the surface of the Earth, we use an object's weight to infer its mass.
Big Ideas: Substances will float or sink relative to each other depending on their relative density to the liquid they are in. Density can be calculated using the mass and volume of a substance.The Density of water = 1 gram/milliliter
One milliliter = One cubic centimeter
Demonstration

How are Coke and Diet Coke different in density?
Coke: What do you think will happen when a can of Coke is placed into a tub of water?
Diet Coke: What do you think will happen when a can of Diet Coke is placed into a tub of water?
What did we learn?
1. What happened to the two cans? The Diet Coke floated, and the regular Coke sank.
2. Which can is more dense? Explain. Regular Coke
3. How could we make the Coke float without touching the can?
4. What did we do to make the Coke float? Why did that work?
By adding salt to the water, we made the water more dense than the Diet Coke, making it float.
How many packets of sugar are in a can of Coke?
Watch the video to find out!
How is density calculated?
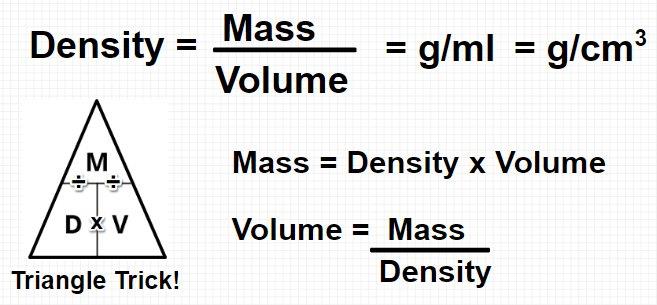
Notes on Density
Density is a derived unit (from mass and volume).
Density is a property of a uniform substance.
Density remains the same regardless of the object's size (under the same conditions).
Temperature and pressure changes affect an object’s density.Hotter temperatures generally cause objects to expand, thereby decreasing their density.
Cooler temperatures generally make objects contract, increasing their density.Higher pressures can compact an object, making it denser.
Lower pressures can allow an object to expand, making it less dense.
Mass can be measured using a scale (because gravity is fairly consistent on earth).

Volume can be calculated if you have a regular-shaped rectangular solid.
Volume = Length x Width x Height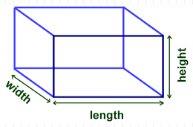
Finding the density of a regularly boxed-shaped object.
5. What is the density of the following object?
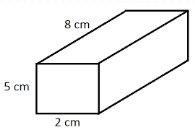
Volume = Length x Width x Height
Volume = 2 cm x 5 cm x 8 cm
Volume = 80 cm³

When weighed on a scale, the mass of this object was found to be 88.0 grams.
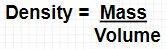
Density = 88.0 grams/80.0 cm³
Density = 1.1 g/cm³
Will it float?
Not in water because it has a density greater than 1.0 g/cm³
Finding the density of an irregularly shaped object.
6. What is the density of the Jade sample?
To find the volume, you must use water displacement.
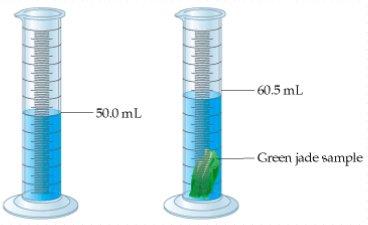
Volume = 60.5 mL - 50.0 mL = 10.5 ml

When weighed on a scale, the mass of the Jade was found to be 34.5 grams.
Density = Mass/Volume
Density = 34.5 grams/10.5 mL
Density = 3.3 g/ml
A Story of Archimedes and the Gold Crown!
Explain in your journal the Story of Archimedes and the Gold Crown!
Summary
*Density is a property describing how compact atoms are in a material.
*The density of water is 1 g/ml.
Things that have a density greater than 1 g/ml will sink, and those that have a density less than 1 g/ml will float.
*To find the density of a material, you need its mass and volume.
*Mass can be found by weighing the material on a scale.
*If the material is box-shaped, you can find the volume by measuring and multiplying the length, width, and height.
*If the material is irregularly shaped, you must use water displacement to find the volume of the material in milliliters.
*Density = Mass/Volume and the units are (g/cm3) or (g/ml).
Bonus: Do the following experiment to create a density column.
Write down your experience, take a photo, and incorporate it into your results.

