-
The Atom!
Stars are atom builders!
But only to a certain extent...
First, we must learn about the sub-atomic particles that make up atoms.
Venus Flytrap may have said it best!
On a Thanksgiving note, one of the funniest episodes ever to run on TV has to be WKRP in Cincinnati S01E07 Turkeys Away!
Everything in the Universe consists of atoms, even you!
- Atoms: the smallest unit that makeup elements
- Atoms are made up of 3 basic subatomic parts.
- Protons: positively charged and located in an atom's center (nucleus). A proton has a +1 electrical charge.
The number of protons determines the name of an element.
Atomic number = the number of protons - Neutrons: neutral or no charge, also located in the nucleus
Protons and neutrons have nearly the same size and mass.
Atoms that have the same number of protons but different numbers of neutrons from each other are called isotopes.
Atomic Mass Number = the number of protons + the number of neutrons - Electrons: negatively charged and orbit the nucleus. An electron has a -1 electrical charge.
Electrons are so much smaller and lighter that they basically don't even affect the mass of an atom.
If you have a different number of protons and electrons in an atom, the atom has an electrical charge and is called an ion.
- Protons: positively charged and located in an atom's center (nucleus). A proton has a +1 electrical charge.
- The nucleus of an atom is extremely dense and contains nearly all the mass of each atom.
Electrons contribute very little mass to the atom and orbit so far away from the nucleus that each atom is 99.9% empty space.
If the atom were the size of a sports arena, the nucleus would be the size of a pearl! - There are over 100 different kinds of atoms.
- Your body has around 7 billion billion billion atoms, yet you replace about 98% of them every year!
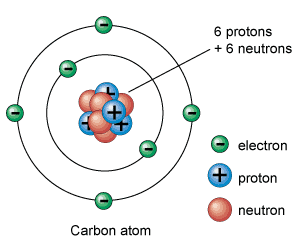
Like every other living creature, you are a carbon-based life form!How many protons? Six protons
How many electrons? Six electrons
How many neutrons? Six neutrons
What is its atomic number? Six
What is its atomic mass? 12
What is it called if the carbon atom has an extra neutron? Isotope
What is it called if the carbon atom has an extra electron? Ion (Anion).
A Cation has a positive charge (missing an electron).
Carbon Atoms
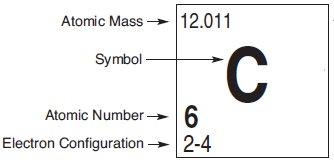
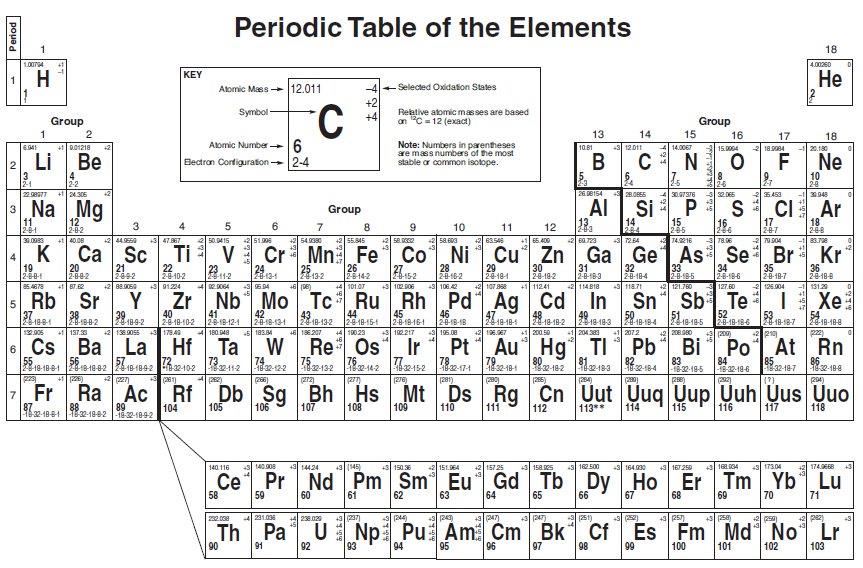
The above table is what you will use if you take Chemistry.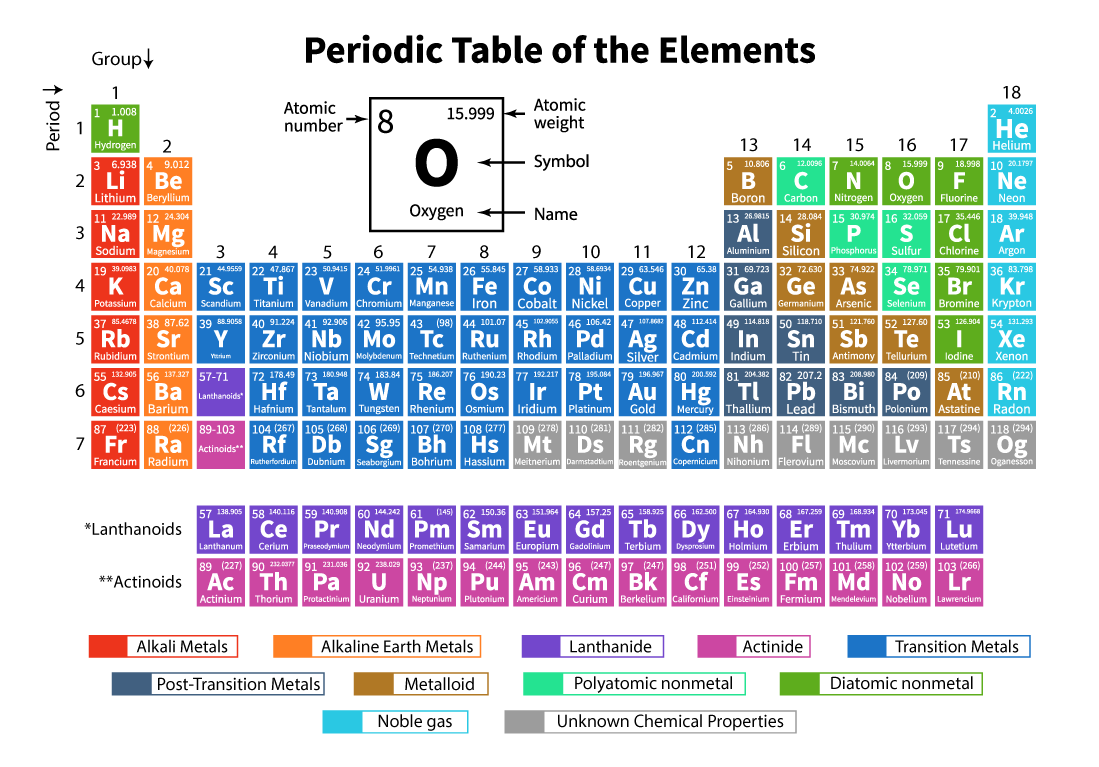
Do the activity located here before you attempt your quiz on atoms.
- Atoms: the smallest unit that makeup elements

