-
Earth Science Journal!
Mineral Identification
Mineral: A natural, inorganic solid with a definite structure and composition.1. Solid - not liquid or gas
2. Naturally Occurring - not made by humans
3. Definite Chemical Composition - ex: SiO2 = Quartz
4. Orderly atomic arrangement - atoms are arranged in geometric patterns (crystalline)
5. Inorganic - not alive, not made from something alive
Copy down the hardness numbers for the following materials. Please take out your reference tables and turn to the back page.
Please take out your reference tables and turn to the back page.Copy down the following definitions.
Luster - how a mineral’s surface reflects light (metallic or nonmetallic)
Streak: the color of the powder of the mineral when scratched on a porcelain plate.
Hardness - a mineral's resistance to being scratched.
Cleavage - how a mineral breaks along planes of weakness, creating flat surfaces.
Fracture - when a mineral breaks irregularly.
Conchoidal fracture: breaks like glass (sharp edges)
LusterMetallic or Non-metallic
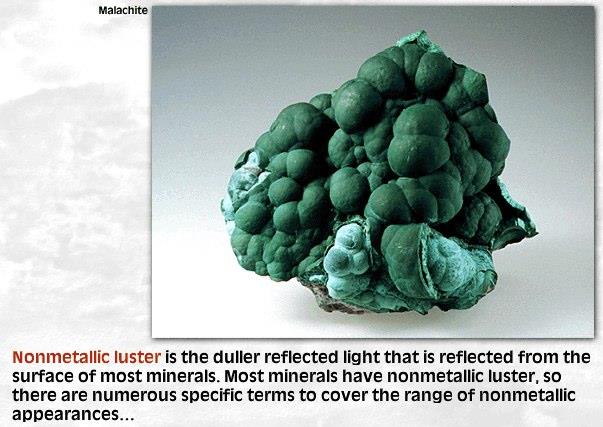

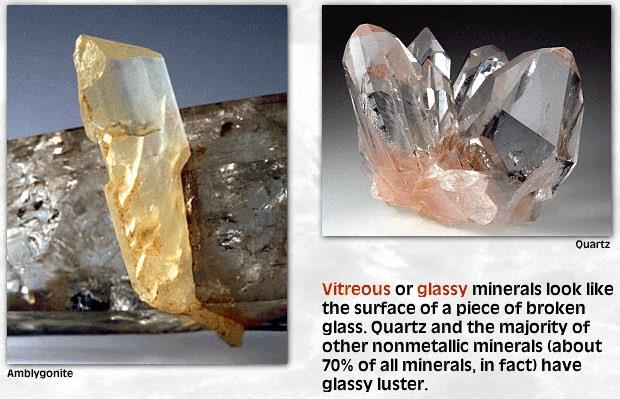 Cleavage or Fracture
Cleavage or Fracture

 Calcite has rhombohedral cleavage!
Calcite has rhombohedral cleavage!
It breaks into rhombus shapes (not at 90 degrees).
Color FluoriteColor is not a good way to identify a mineral!
FluoriteColor is not a good way to identify a mineral!
*The same mineral may be many different colors.
*Different minerals can be the same color.
*The color of a mineral can change when it is weathered.
Streak


Other Characteristics Acid- Calcite and powdered dolomite will effervesce (fizz) in dilute hydrochloric acid (HCl)Taste- Halite is rock salt and will taste salty. Please don't taste the minerals. I will let you know if it is salty.Magnetism- Attracted by a magnet. Some magnetite will pick up paper clips.Fluorescence- some minerals (mostly forms of calcite) will glow in fluorescent colors under black (UV) light.Double refraction- some clear forms of calcite (Iceland Spar) will make a double image of words.
Acid- Calcite and powdered dolomite will effervesce (fizz) in dilute hydrochloric acid (HCl)Taste- Halite is rock salt and will taste salty. Please don't taste the minerals. I will let you know if it is salty.Magnetism- Attracted by a magnet. Some magnetite will pick up paper clips.Fluorescence- some minerals (mostly forms of calcite) will glow in fluorescent colors under black (UV) light.Double refraction- some clear forms of calcite (Iceland Spar) will make a double image of words.
Tape the classification scheme into your journal.
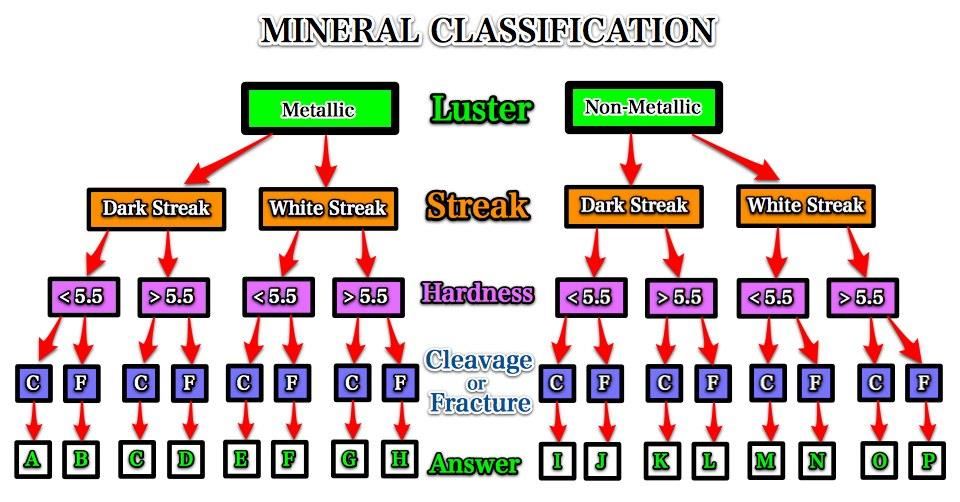 Use the above classification scheme to identify the minerals 1-5 by letter.
Use the above classification scheme to identify the minerals 1-5 by letter.Mineral Number Mineral Letter 1 2 3 4 5
Some minerals glow in the dark!
The Silicates!Any minerals that are made from the silicon-oxygen tetrahedron
Look at the back of your reference tables and write down five silicate minerals.Talc, Muscovite mica, Biotite mica, Pyroxene, Amphibole, Potassium feldspar, Plagioclase feldspar, Olivine, Quartz, Garnet.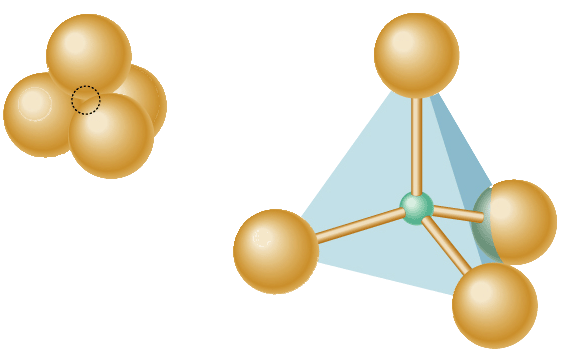
Silicon-oxygen tetrahedron The envelope pyramid!
These are the most abundant minerals on earth, and all are made from the silica-oxygen tetrahedron.
What is this?How about this thing?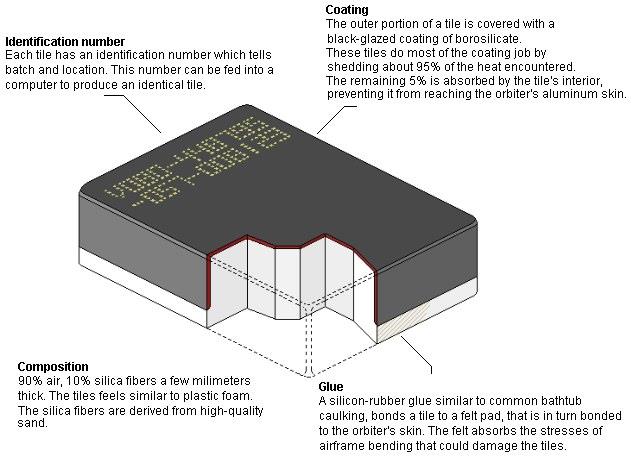
What is this?

Columbia Disaster!Reflection: Explain the most important things that you learned about classifying minerals.
BonusWatch the movie NOVA "Origin of Life" and explain how minerals have changed the Earth and are connected to life on Earth.Origin of Life - How Life Started on Earth
https://youtu.be/QE5Js-9AzHo?si=im2NkK10fgFIEKlz
How did the Earth go from a black Earth to a green Earth?
---------------------------------Mineral Number Mineral Letter 1 D 2 M 3 A 4 O 5 P

